Discover new things – your knowledge advantage with us.
Welcome to our blog! Here we share exciting news, inspiring case studies, and practical knowledge on topics that move your industry. With our posts, we aim not only to inform but also to provide food for thought and support you in making informed decisions. Dive into a world full of expertise, innovation, and insights that truly matter.
Quickly find what interests you.
Top White Papers at a Glance
This white paper reveals how Artificial Intelligence is revolutionizing food production. Discover why AI-driven inspection systems are a game-changer for the industry's future.
This white paper explores advancements in AI-driven sorting technology and demonstrates how you can leverage these innovations to make your processes more precise, sustainable, and profitable.
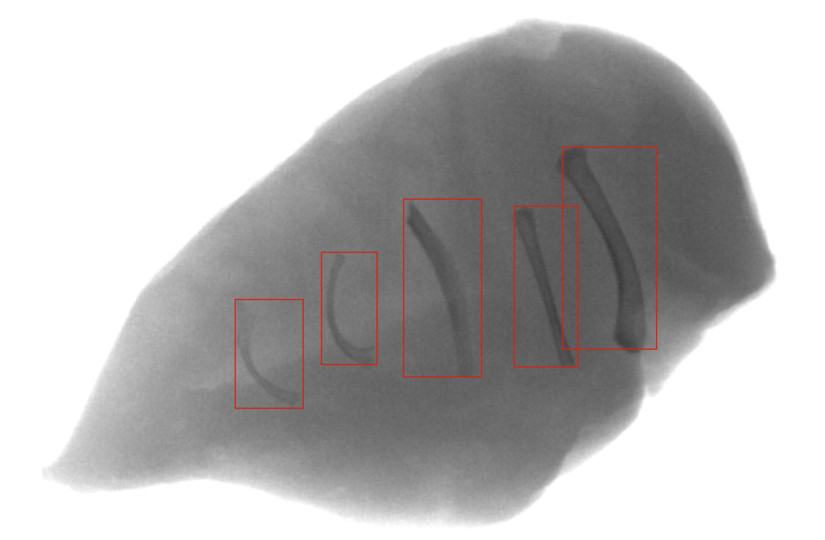
This white paper explores various maintenance strategies with a focus on Predictive Maintenance, offering insights into how AI-driven predictive maintenance can enhance your production efficiency and boost food safety.
Discover what truly matters in recycled materials and the essential steps to create a future-proof process landscape for their use in this white paper.




.JPG)



.JPG)
.jpg)





.JPG)

.JPG)


.jpg)


.jpg)
.jpg)

.jpg)










.jpg)
.jpg)
.jpg)


.jpg)


.jpg)









.jpg)






.jpg)

.jpg)








.JPG)






.jpg)










.jpg)


.jpg)






.jpeg)




.JPG)
